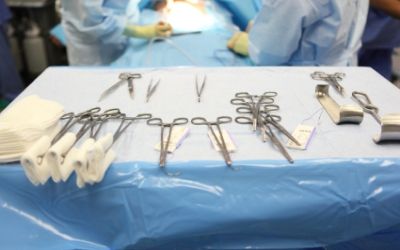
Exemplary Terminally Sterilized Medical Devices
Terminal sterilization refers to a sterility assurance level SAL6 of 106 SAL6 is considered the standard for medical devices 240 and describes the process that ensures that the medical devices and implants are sterile at the point of use.
Terminally sterilized medical devices. ISO 10993-42006 - Biological evaluation of medical devices Part 4. Applications of Cleaning Techniques 2019. Medical devices that are terminally sterilized.
Vaporized hydrogen peroxide abbreviated VH2O2 by. These processes include. Experience in microbiology sterilization validation terminal aseptic andor reprocessing and environmental controls for a Medical Device or Pharmaceutical manufacturing facility is required.
The bioburden of most medical devices generally is a lesser challenge to the sterilization process than biological indicators and overkill cannot be demonstrated. Terminal sterilization plays a vital role in the provision of safe medical devices. The main changes compared to the previous edition are.
DEPARTMENT OF HEALTH HUMAN SERVICES Public Health Service AUG I 82008. Buy this standard Abstract Preview. Literature shows that about fifty percent 123 of all sterile medical devices in the US.
These processes include forming sealing and assembly of preformed sterile barrier systems sterile barrier systems and packaging systems. Reusable Medical Equipment Device or Item. Packaging Validation - New Machinery - Terminally Sterilized Medical Devices.
Experience in microbiology sterilization validation terminal aseptic andor reprocessing and environmental controls for a medical device or pharmaceutical manufacturing facility. Terminal sterilization plays a vital role in the provision of safe medical devices. Are sterilized with ethylene oxide.